NEJM:Sofosbuvir可有效治疗基因2型或3型HCV感染
| 导读 |
对于聚乙二醇干扰素治疗无效,或既往对干扰素治疗无应答的基因2或3型丙肝病毒(HCV)慢性感染者,目前还没有有效的治疗选择。在2期临床试验中,包含口服核苷酸聚合酶抑制剂sofosbuvir的治疗方案表现出对2或3型HCV感染者的有效性。美国佛罗里达大学的 Nelson博士及其同事日前发表于国际权威杂志NEJM的研究显示,对于聚乙二醇干扰素和利巴韦林治... |
对于聚乙二醇干扰素治疗无效,或既往对干扰素治疗无应答的基因2或3型丙肝病毒(HCV)慢性感染者,目前还没有有效的治疗选择。在2期临床试验中,包含口服核苷酸聚合酶抑制剂sofosbuvir的治疗方案表现出对2或3型HCV感染者的有效性。美国佛罗里达大学的 Nelson博士及其同事日前发表于国际权威杂志NEJM的研究显示,对于聚乙二醇干扰素和利巴韦林治疗无效的HCV 2型和3型感染者,sofosbuvir 和利巴韦林12周或16周治疗方案是有效的。相对而言,该方案对感染2型HCV病毒或无肝硬化的患者疗效愈加显著。对于经既往治疗的3型感染者,16周治疗方案显著优于12周治疗方案。
研究人员展开了两项随机3期临床试验,纳入慢性HCV基因2或3型感染者。在第一项试验中,聚乙二醇干扰素 (peginterferon)治疗无效的患者接受口服sofosbuvir和利巴韦林(207例患者)或对应安慰剂(71例患者)治疗12周。在第二项试验中,既往干扰素治疗无应答的患者接受sofosbuvir 和利巴韦林治疗12周(103例患者)或16周(98例患者)。主要终点为治疗后12周的持续病毒学应答率。
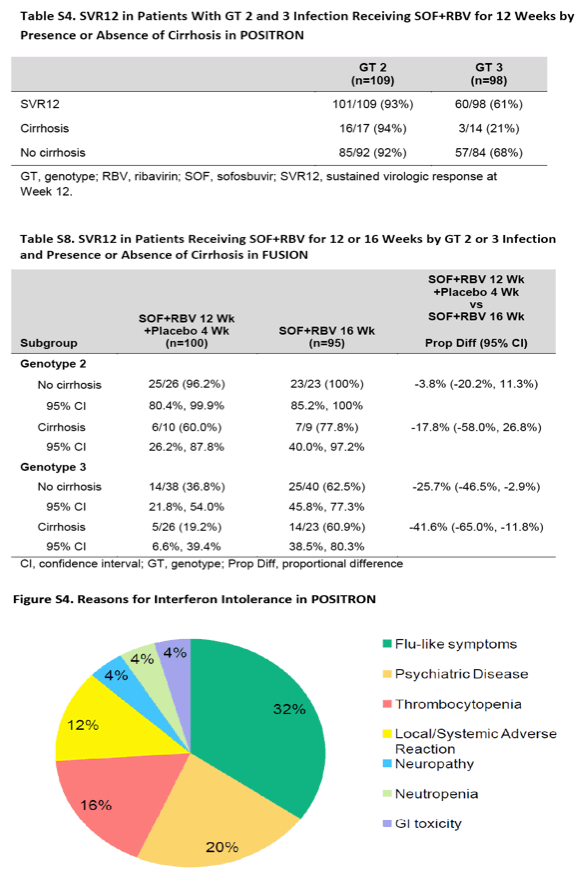
结果显示,聚乙二醇干扰素治疗无效的患者人群中,使用sofosbuvir和利巴韦林治疗后,持续病毒学应答率为78% (95% 置信区间[CI],72%-83%),与之相比,安慰剂治疗组的这一数据为0% (P<0.001)。在经既往治疗的患者人群中,经过12周治疗后应答率为50%,经16周治疗后应答率为73%(差异,-23%;95% CI,−35 to −11;P<0.001)。在两项研究中,基因3型HCV感染者较2型感染者的应答率要低,肝硬化患者人群的应答率较无肝硬化者低。研究发现,最常见的不良反应事件为头疼、疲劳、恶心、以及失眠;sofosbuvir 组中断治疗的比例低(1%-2%)。
研究人员由此得出结论,对于聚乙二醇干扰素和利巴韦林治疗无效的HCV 2型和3型感染者,sofosbuvir 和利巴韦林12周或16周治疗方案是有效的。相对而言,这一治疗方案对感染基因2型HCV病毒或无肝硬化的患者疗效愈加显著。对于经既往治疗的基因3型HCV感染者,16周治疗方案显著优于12周治疗方案。
相关链接:
Sofosbuvir联合利巴韦林治疗HCV基因2、3型患者的病毒学应答率达78%。Gilead科技公司在2012年11月27日宣布了其POSITRON三期研究数据,为期12周的试验,每日1次sofosbuvir(之前名为GS-7977)联合利巴韦林(RBV)用于干扰素(IFN)治疗无效的2或3型HCV感染者。该研究发现78%的患者(161/207)在治疗完成12周后,依然无法检测到其HCV RNA(SVR12)。Sofosbuvir的安全性特征与前期研究结果相似,且鲜有因不良事件而终止治疗的患者。能在三分之二的患者中实现持续病毒学应答,这对于那些没有合适替代疗法的患者来说,是一个让人欣喜的结果。这些患者之前都被确定拒绝了干扰素疗法,或不能接受,或对干扰素不耐受。
在POSITRON研究里,那些携带有2或3型基因型HCV,且对干扰素不适合,不耐受或不接受的患者,以3:1比例被随机分配,接受为期12周的sofosbuvir(400mg,每天一次)联合RBV(每天两次,剂量依体重而定)治疗(n=207),或接受安慰剂治疗(n=71)。在这些患者中,15%患有代偿性肝硬化,53%为2型HCV感染者。在2型和3型HCV携带者中,SVR12分别为93%和61%。接受安慰剂治疗的患者没有一例达到SVR12。超过10%的患者中出现不良反应,最常见的包括:疲劳、恶心、头痛、失眠、瘙痒和贫血。
原文链接:
Sofosbuvir for Hepatitis C Genotype 2 or 3 in Patients without Treatment Options
Background
Patients chronically infected with hepatitis C virus (HCV) genotype 2 or 3 for whom treatment with peginterferon is not an option, or who have not had a response to prior interferon treatment, currently have no approved treatment options. In phase 2 trials, regimens including the oral nucleotide polymerase inhibitor sofosbuvir have shown efficacy in patients with HCV genotype 2 or 3 infection.
Methods
We conducted two randomized, phase 3 studies involving patients with chronic HCV genotype 2 or 3 infection. In one trial, patients for whom treatment with peginterferon was not an option received oral sofosbuvir and ribavirin (207 patients) or matching placebo (71) for 12 weeks. In a second trial, patients who had not had a response to prior interferon therapy received sofosbuvir and ribavirin for 12 weeks (103 patients) or 16 weeks (98). The primary end point was a sustained virologic response at 12 weeks after therapy.
Results
Among patients for whom treatment with peginterferon was not an option, the rate of a sustained virologic response was 78% (95% confidence interval [CI], 72 to 83) with sofosbuvir and ribavirin, as compared with 0% with placebo (P<0.001). Among previously treated patients, the rate of response was 50% with 12 weeks of treatment, as compared with 73% with 16 weeks of treatment (difference, -23 percentage points; 95% CI, -35 to -11; P<0.001). In both studies, response rates were lower among patients with genotype 3 infection than among those with genotype 2 infection and, among patients with genotype 3 infection, lower among those with cirrhosis than among those without cirrhosis. The most common adverse events were headache, fatigue, nausea, and insomnia; the overall rate of discontinuation of sofosbuvir was low (1 to 2%).
Conclusions
In patients with HCV genotype 2 or 3 infection for whom treatment with peginterferon and ribavirin was not an option, 12 or 16 weeks of treatment with sofosbuvir and ribavirin was effective. Efficacy was increased among patients with HCV genotype 2 infection and those without cirrhosis. In previously treated patients with genotype 3 infection, 16 weeks of therapy was significantly more effective than 12 weeks. (Funded by Gilead Sciences; POSITRON and FUSION ClinicalTrials.gov numbers, NCT01542788 and NCT01604850, respectively.) When studied in clinical trials, the current standard-of-care therapy for patients with hepatitis C virus (HCV) genotype 2 or 3 infection - pegylated interferon in combination with ribavirin for 24 weeks - resulted in a sustained virologic response in 70 to 85% of patients who had not received prior treatment and in 55 to 60% of those who had received treatment.1-4 However, a substantial proportion of patients with HCV infection remain untreated owing to absolute or relative contraindications to interferon therapy, such as hepatic decompensation, autoimmune disease, and psychiatric illness.5 In addition, interferon causes a range of constitutional symptoms or hematologic abnormalities that may require discontinuation of therapy in a considerable number of patients.6 Some patients decide against interferon therapy for a variety of reasons, including aversion to injections and anxiety about the adverse events associated with treatment. Moreover, the 15 to 30% of patients with HCV genotype 2 or 3 infection who do not have a response to interferon therapy have no alternate therapeutic options. These populations - which by one estimate constitute the majority of patients infected with HCV5 - are in need of effective treatments.
Sofosbuvir is an oral nucleotide analogue inhibitor of the HCV-specific NS5B polymerase with in vitro activity against all HCV genotypes.7 In a phase 2 study of treatment for 12 weeks with sofosbuvir and ribavirin in patients with HCV genotype 2 or 3 infection, 10 of 10 previously untreated patients (100%) and 17 of 25 previously treated patients (68%) had a sustained virologic response.8,9 This oral regimen had an acceptable safety profile, with no premature discontinuations of sofosbuvir therapy owing to adverse events.9
In this article, we present the results of two phase 3 trials of treatment with sofosbuvir and ribavirin in patients with HCV genotype 2 or 3 infection for whom treatment with peginterferon was not an option and in those who did not have a response to prior interferon treatment. The primary objective of both studies was to evaluate the efficacy of sofosbuvir and ribavirin, as measured by the proportion of patients with a sustained virologic response at 12 weeks after the end of treatment, and to evaluate the safety of this regimen.
关注转化医学网
【转化医学网 新浪微博:@转化医学网】
【转化医学网 公众微信账号:zhuanhuayixue】或扫描二维码关注微信

扫描微信二维码关注
来源:丁香园 腾讯登录
腾讯登录
还没有人评论,赶快抢个沙发